Sterilization of liquids, solids, waste in disposal bags and hazardous biological substances
Sterilization of liquids, solids, waste in disposal bags and hazardous biological substances
STERILIZATION OF LIQUIDS, SOLIDS, WASTE IN DISPOSAL BAGS AND HAZARDOUS BIOLOGICAL SUBSTANCES

Sterilization that is easy, safe, accurate, reproducible and validatable.
he sterilization process in an autoclave can be rather difficult. For example, when sterilizing liquids or solids (instruments, glassware, filters or textiles) for later use in the lab, the sterilization process must ensure to produce a sterile product, reproducible at any time. Products sterilized for use in labs cannot be tested for sterility, as this would contaminate them again and thus they could not be used in the lab any longer. Validation of steam sterilization processes has become an increasingly important issue to ensure reproducible results that can be verified. Furthermore, safety aspects must generally be considered when autoclaving, and in particular when sterilizing liquids. Sterilization is generally done at a temperature of 121⁰C. This corresponds to a steam pressure of approx. 2 bar. Such high temperatures and the ensuing pressure may pose considerable risk potential for the operator, if the autoclaving process has design flaws or is not executed properly.
Sterilization of liquids and liquid waste in bottles
Sterilizing liquids is one of the most demanding tasks in the lab. Sterilization processes may take a very long time, bottles must be open or at least vented, part of the liquid will boil away, liquids may boil over and bottles may even burst. Another issue to be addressed is, whether the liquids inside the bottles even reach the required sterilization temperature (e.g. 121°C), and when they may be safely taken out of the autoclave after completion of the sterilization process.
Viewing the sterilization process for liquids, it splits into three phases:
1. Heating phase and equilibration time (H)
2. Sterilization phase, e.g. 121⁰C for 20 minutes (S)
3. Cooling-down phase to a safe removal temperature (C)
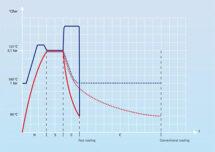
Illustration 1:

Blue line: Temperature inside the chamber (pressure vessel) of the autoclave
Red line: Temperature measured inside the liquid
Illustration 1 details the individual phases by means of a graphic display. The blue line represents the temperature inside the pressure vessel of the autoclave, the red line the temperature inside the liquid. It is clearly visible that the temperature inside the pressure vessel of the autoclave reaches the required temperature of 121⁰C quickly, whereas the liquids inside the bottles need a much longer time to reach sterilization temperature. During the heating-up time, thermal energy of steam is transferred to the bottles by means of condensation of the steam. This condensation process and the ensuing thermal transfer require quite some time, explaining the time lag between the mere heating-up of the pressure vessel and the heating-up of the liquid itself. The time required to achieve the same temperatures inside the pressure vessel of the autoclave and inside the liquids is called equilibration time.
Many of the autoclaves used in labs today are still not equipped with a temperature measurement inside a reference vessel. Thus, the exact temperature of the liquid to be sterilized is not being measured and cannot be used to regulate the sterilization process. These autoclaves start the sterilization phase after the required temperature inside the pressure vessel of the autoclave has been reached. The equilibration time required for the liquid to also reach the required temperature is not taken into consideration. The liquids thus never reach a sterilization temperature of e.g. 121°C and consequently, the biological efficiency of the sterilization process is no longer ensured. Depending on the resistance of the micro-organisms to be deactivated, they are only deactivated in part or not at all.
Measuring the temperature inside a reference vessel
By measuring the temperature inside a reference vessel by means of a temperature sensor, the exact temperature of the liquid to be sterilized can be determined and then used to regulate the sterilization process. Sterilization time starts only, after the required sterilization temperature inside the liquid has been reached.
The reference vessel is to be filled with water. It is crucial, that size and filling level of the reference vessel correspond to the largest vessel filled with the liquid to be sterilized.
Safe removal temperature

The temperature sensor for measuring inside the reference vessel is required to ensure that the sterilization temperature inside the liquid is reached. It is also required to ensure a safe removal temperature after the sterilization has been carried out. Inside an autoclave, liquids are heated up to temperatures considerably above the regular boiling point (100⁰C). The thermal heat transferred into the liquid in connection with the associated excess pressure may cause considerable hazards for the operator of an autoclave. For example, a delayed boiling may occur which means that the liquid will spontaneously start to boil when the autoclave is opened. This instantaneous boiling will generate a pressure wave consisting of steam and hot liquid, erupting – similar to a geyser – from the vessels. 1 liter water will generate 1000 liter steam! Based on this considerable hazard potential, autoclaves used for sterilizing liquids are subject to corresponding regulations. DIN EN 61010-2-040 stipulates that autoclaves used for sterilizing liquids must be equipped with safety devices preventing an opening of the autoclave before the liquids have not been cooled down to a removal temperature safe for the operator. A safe removal temperature is defined in the standard to be 20K below the boiling point of water at atmospheric ambient pressure. This corresponds to a safe removal temperature of 80⁰C. State-of-the-art autoclaves are equipped with a temperature and pressure dependent door lock. That prevents the autoclave from being opened, when the pressure vessel is pressurized and for as long as the temperature measured inside the liquid exceeds the stipulated 80⁰C.

Cooling liquids down to a safe removal temperature may take a rather long time. A size frequently used for autoclaves in labs is an autoclave with a pressure vessel capacity of approx. 150 liter. Is such an autoclave filled with bottles containing the liquid to be sterilized, the total sterilization cycle may take up to 10 hours. I.e., during one working day, not even one sterilization process may be completed. It is thus recommended to equip the autoclave with a recooling system considerably reducing the overall time required to sterilize the batch and preventing further hazards and disadvantages when sterilizing liquids.
Quick recooling – maximizing productivity and safety
Recooling systems available for autoclaves basically distinguish between two types of cooling systems:
1. Cooling by evaporation – boiling the liquid during the cooling phase
2. Cooling by radiation – heat radiating from the liquid, no boiling of the liquid with this cooling system
Cooling by evaporation is the most frequently used type of cooling inside an autoclave. That may be:
– Self-cooling via slowly releasing steam
– Ventilation cooling – cold ambient air is ventilated onto the pressure vessel from the outside
– Water cooling without support pressure
All cooling types stated above have serious disadvantages when sterilizing liquids and may contain considerable hazard potential, if the sterilization process is not carried out properly, as this type of cooling requires the liquid to be cooled down to boil.
1. When the liquid boils during the cooling phase, part of the liquid is lost. The loss of liquid to be expected usually ranges between 3 and 10 %, but may be considerably higher – depending on the contents of the liquid. Especially, if the protein content of the liquid is high, it tends to boil even more, increasing again the loss of liquid.
2. As the liquids must boil to cool down, the probability for them to boil over is high. Therefore, the bottles are only being filled half or even only one third to prevent over boiling. This is, on one hand a considerable loss in productivity, as 50-70 % of the capacity available (inside the bottles) is lost. On the other hand, boiling over cannot be prevented reliably. If liquids boil over, the autoclave must be cleaned and, for example, agar-based liquids may flow into the pipe system (drain) of the autoclave and block it, when the agar cools down and solidifies there. Cleaning the pipe system is frequently highly cost-intensive and only possible for the manufacturer of the autoclave.
3. Liquids can only boil from open bottles. Therefore, the bottles must be open or at least vented (the lid must be slightly open). If venting the bottles is forgotten or done improperly, the liquid inside cannot boil during the recooling phase and thus will not cool down. After the reference vessel has reached the cooling temperature of 80⁰C and thus allows to open the autoclave, the hermetically closed bottles are still on sterilization temperature with the corresponding pressure of e.g. 121⁰C, 2 bar. This poses a considerable hazard, as these bottles may explode during removal from the autoclave and the liquid contained therein may evaporate spontaneously – similar to a delayed boiling. 1 liter water generates 1000 liter steam!
It is therefore recommended when procuring an autoclave to define exactly what application/s it will be used for and how it should be equipped with regards to productivity and safety.

Cooling by radiation (Quick cooling with support pressure)
Cooling by radiation has considerable advantages as compared to cooling by evaporation. During quick cooling with support pressure, the pressure vessel is cooled down across its entire surface via externally attached cooling coils containing cold water. Before cooling is activated after the sterilization phase, the steam inside the pressure vessel is replaced by sterile-filtered compressed air. This pressurized air reliably prevents the liquid from boiling during the cooling phase. Heat is transferred from the liquid to the cold walls of the pressure vessel by means of radiation and the liquids thus are cooled down.
Quick cooling with support pressure allows for a substantial gain in productivity, as process time compared to self-cooling is considerably reduced. Whereas self-cooling requires up to 10 hours for an overall autoclaving process, recooling time by means of quick cooling with support pressure may be reduced by up to 60 % – depending on the quantity of liquids processed.
Furthermore, all hazards and disadvantages described for cooling by evaporation (delayed boiling, loss of liquid, over boiling, no cooling of hermetically sealed bottled) are reliably eliminated, as the liquid does no longer boil. This type of cooling allows for the bottles to be filled up to their maximum filling level (50 to 70 % productivity gained) – even hermetically sealed bottles can be used. Opening or venting the bottles is no longer required.
Further optimize your process cycles
State-of-the-art autoclaves allow to further optimize the cooling of liquids in modules. This further increases productivity but also influences the quality of the liquids to be sterilized. Many liquids contain ingredients that are not very heat-stable. The liquids must be sterilized, but the time they will be exposed to heat impact, should be as short as possible so that heat-labile ingredients do not suffer negative impacts.
Module 1 – Radial ventilator
The radial ventilator generates an airstream inside the pressure vessel of the autoclave during the cooling phase. This airstream forces the heat from the bottles onto the walls of the pressure vessel cooled by quick cooling with support pressure. This process will shorten the cooling time by up to 70 % compared to self-cooling.
Module 2 – Ultracooler
The Ultracooler is an additional water-cooled heat exchanger, integrated directly into the pressure vessel of the autoclave. This allows to remove the heat from the bottled exactly there where the heat is. Inside the pressure vessel. By means of a substantially improved thermal transfer, cooling time can be reduced by up to 90 % as compared to self-cooling.
Note: As radial ventilator and Ultracooler are installed inside the pressure vessel, take care that they will not reduce the usable space available inside the autoclave.
Sterilizing solids and waste in destruction bags
When sterilizing solids (e.g. instruments, empty glassware, pipette tips in boxes, filters and textiles) as well as when decontaminating waste in destruction bags it must be ensured that a steam atmosphere is building exactly where it is required. Namely on all inner and outer surfaces of the product to be sterilized. Many autoclaves do not remove air reliably from the autoclave and from the product. If air remains inside the autoclave and the product, no sterilizing effect is possible, as only steam transports the thermal energy required to reliably deactivate microorganisms.
Ineffective air removal
Illustrations 5 and 6 show an ineffective air removal using the example of a box with pipette tips as well with destruction bags. If the autoclave is just simply heated up, air is displaced and a steam atmosphere builds up inside the pressure vessel of the autoclave but air remains inside the product to be sterilized. Air remaining inside the product, however, prevents the steam from entering where its thermal energy is needed to achieve a sterilizing effect.
At the same temperature as steam (e.g. 121°C), air contains only a fraction of the required thermal energy. For products that cannot be sterilized within a steam atmosphere, there are hot-air

sterilizers – however, they sterilize at higher temperatures (180 – 250⁰C) requiring a lot more time (up to several hours sterilization time). A sterilizing effect of air at temperatures of 121°C – 134°C and a sterilization time of 3-20 minutes that are usually used inside a steam sterilizer is therefore in praxis not given.
Effective air removal

For a complete and reproducible removal of air from the autoclave and the product to be sterilized, a fractionated pre-vacuum is to be used. Therefore, the autoclave is equipped with a vacuum system. During the heat-up phase, vacuum cycles take place for effective air removal followed by steam injections. Usually, a threefold fractionated pre-vacuum is applied – depending on the product, however, even more fractions may be necessary.
Drying of solid objects – Superdry

Solids such as instruments or empty glassware are usually put inside a drying oven following the sterilization process. State-of-the-art autoclaves allow for drying solids directly after the sterilization process. Sterilization and drying in one process. Further handling of the sterilization material possibly causing recontamination is not necessary.
Sterilization of biologically hazard substances
Sterilization of biologically hazardous substances is a special challenge. During the heating-up phase, the air inside the autoclave is replaced with steam. Air is displaced from the autoclave and released into the room where the autoclave is installed. TRBA 100 – Technical Regulations for Biological Working Materials requires that, in labs from Security Level BSL2, process exhaust air from an autoclave must be treated as the exhaust air may be contaminated by microorganisms from the product to be sterilized. An appropriate process must be used. In the case of autoclaves, it is usually filtration. Therefore, the autoclave can be equipped with an air exhaust filter. All air displaced from the autoclave passes the filter with microorganisms being retained in the filter. The filter is sterilized “in-line” during the sterilization process to deactivate the micro-organisms retained therein.
TRBA 100 deals only with exhaust air released from the autoclave, but not the condensate collected. During the sterilization process, steam condenses on the product and thus turns again into water (condensate). This water may also be contaminated with microorganisms. Therefore, the condensate must remain inside the autoclave during the sterilization process and must also be sterilized “in-line” as well before being drained after successful sterilization.
Qualification and validation
During qualification it is verified, whether a device is suitable for its intended use and whether a process – e.g. a sterilization process – can be performed considering the product to be sterilized with a continuing (reproducible) result, a sterile product. Generally, the qualification process is split into three basic parts:
1. IQ – Installation Qualification
Verification, whether a device was manufactured and installed according to specifications.
2. OQ – Operational Qualification
Verification, whether a device operates generally according to specified functions.
3. PQ – Performance Qualification
Verification, whether a device with product to be treated functions according to specificationsTarget of Qualification and Validation is a documented proof that a device is suitable for its intended use.
Target of Qualification and Validation is a documented proof that a device is suitable for its intended use.
The sterilizing effect of an autoclave process is verified during OQ (empty chamber) and PQ (with product) by means of external data loggers for temperature and pressure as well as using bio-indicators based on Bacillus Stearothermophilus. Whereas external data loggers for temperature and pressure provide evidence that the control of the autoclave displays and documents reliable values as well as performs the sterilization process within defined tolerances, bio-indicators provide evidence as to biological efficiency. To place bio-indicators, it must be defined for which areas of the product to be sterilized it is most difficult to achieve biological efficiency. Accurately in those areas, bio-indicators must be placed to cover a “worst-case scenario“. All steps of an IQ, OQ and PQ must be documented in detail. In each and every case of carrying out an IQ, OQ and PQ, close cooperation between user and manufacturer is required.